The Gut Microbiome
Introduction
The microbiome (microflora or microbiota) consists of trillions of microorganisms (bacteria, fungi, protozoa, viruses) that colonize the skin, nose, digestive system, lungs, and vagina but the most substantial quantity is in the colon and small intestine.
Even the microbiome is known as a "supporting organ," since it plays critical roles in maintaining our body's physiological functions.
At the intestinal stage, the microbiome contains at least 1,000 different bacterial species with more than 3 million genes (150 times more than human genes) and weighing up to 2 kg.
One-third of the gut microbiome is common to most people, while two-thirds are unique to each of us. In other words, it's like an individual identity card, just like the fingerprint, which is unique.
Until birth, babies are practically sterile, having only their body cells. But in the first few years of life, the growing child takes microbes from various sources:
- Initially, the first microbes are inherited from the mother through natural birth and breastfeeding.
The amount and type of bacteria inherited, depend only on the species that exist in the mother.
These early bacteria, with which the baby is colonized, seem to help him fight against allergies, asthma, inflammatory bowel disease, obesity, and diabetes.
Several studies have linked conception by Caesarean region with possible infections of the later adult, and lack of breastfeeding. It is therefore advised that the baby be breastfed wherever possible and as long as possible.
2. Subsequently, through exposure to microbes in the atmosphere (playgrounds, dust, pets, clothes, stress, and pollution) and by diet, which can change the human microbiome either in a way that is beneficial to health or in a risk factor for different conditions.
The "obsession of cleanliness," i.e., the exaggerated concern for the sterilization and disinfection of all objects with which a child or an adult comes into contact, may have a negative impact on health in the short and long term.
Intestinal Microflora functions
The tasks of the intestinal microflora are multiple and complex, and researchers are still studying them and discovering remarkable stuff.
Among other things, the microbiome is involved in:
Absorption. We know what we're eating is extremely important, but more importantly, what we're absorbing. The central place of absorption (90 percent) of the nutrients in the blood is at the level of the small intestine villi, inhabited by bacteria.
The latter contributes to the fragmentation of food into small molecules that will be absorbed. The healthier and more diverse the intestinal flora, the more balanced the absorption of nutrients without any of the disease.
The microbiome also helps to break down complex carbohydrates (starch and dietary fiber) into short-chain fatty acids through the digestive enzymes it secretes.
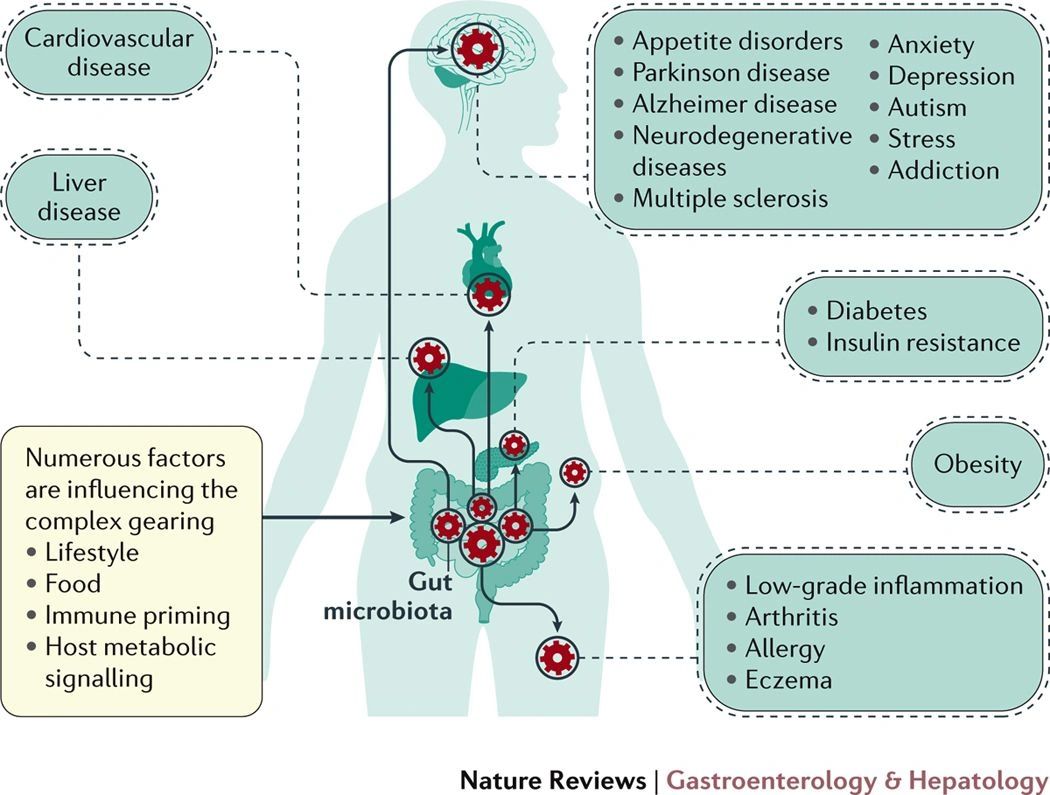
They are involved in the prevention of many chronic diseases, including inflammatory bowel disease (irritable bowel syndrome, Crohn's disease, ulcerative colitis, etc.), cancer, diabetes, cardiovascular disease, etc.
Prevention of autoimmune diseases (rheumatoid arthritis, inflammatory bowel disease, dermatitis).
It appears that the microbiome regulates the increased permeability of the intestinal mucosa, thereby preventing the circulation of large molecules responsible for the onset of autoimmune disorders.
Production of neurotransmitters.
Bacteria in the intestines produce substances that influence our state of happiness or sadness, anxiety, or depression by communicating directly with the human brain.
Studies have confirmed that the microbiome affects the emotional state.
It has also been found that the microbiome is less diverse in people with depression, which alters brain activity.
Modulation of the immune system.
It seems that 70% of the immune function is in the intestine. Bacteria break down potentially toxic compounds that we consume with food and induce the formation of antibodies (cells that battle invaders). Therefore, the immune system can be improved by improving intestinal flora.
Modulation of gene expression.
This suggests that, for example, even though we were born with a genetic predisposition to obesity, the development of good habits that promote the output of a balanced microbiome can decide the recessiveness of the genes for obesity and, at the same time, the superiority of those who provide nutrients and safety.
This is excellent news, which means that biology can not determine our weight fluctuations solely, but on the opposite, we affect our genes.
Absorption, but also the synthesis of some vitamins (vitamin K, vitamin B). For example, the main enzymes needed to make up vitamin B12 are present only in bacteria, the human intestinal flora producing vitamin B12 (in small quantities).
Cancer prevention. Cultivating good bacteria in the intestine is the first positive thing we can do to prevent and monitor the development of cancer, but also other diseases.
Reduction of chronic inflammation. Chronic inflammation is the cause of most cardiovascular diseases, autoimmune diseases, allergies, but also neuronal, renal, hepatic, and osteoarticular disorders.
Influence of body weight by modulating the absorption of fat, regulation of appetite, and control of blood sugar.
Bacterial diversity and the balance between good and bad are some of the most critical aspects of bodyweight modeling.
Obese people have been shown to have a reduced diversity of intestinal bacteria and, at the same time, an increased prevalence of Firmicutes bacteria.
On the other hand, average body weight was associated with a higher number of Bacteroid organisms.
Factors that imbalance the intestinal microbiome
Diet is the element that has a permanent nature of disruption or rebalancing.
The microbiome is made up of microorganisms that can be both beneficial and potentially harmful.
In a healthy body, the two groups coexist without any problems.
Nevertheless, when this equilibrium is disrupted – caused by infectious diseases, other foods, medications, surgery or excessive use of antibiotics – intestinal dysbiosis (which has the following symptoms: stomach problems, diarrhea, vomiting, halitosis, bloating and rash) and the sensitivity of the body to diseases.
A diet rich in processed sugars and, in particular, low in dietary fiber, consumption of carbonated drinks, burgers, fast food, high-fat trans foods (pastry, cookies, muffins)-all of which have a detrimental effect on the human intestinal population.
On the other side, an antibiotic warning signal should be activated. We harm some of the microorganisms in the stomach, oral and vaginal cavities, contributing to the increased production of others.
Therefore, infections such as candidiasis or diarrhea triggered by Clostridium difficile occur.
In order to prevent or treat these infections, it is recommended that substances with probiotic and prebiotic effects should be administered by diet or dietary supplements during antibiotic treatment and at least two weeks after that.
How do we balance the gut microbiome?
While research is still ongoing, a general agreement has been reached that the best way to balance intestinal flora is through a diverse and balanced diet, rich in vegetables, fruits, whole grains, and seeds.
Foods, in themselves, contain either bacterial nutrients or certain bacterial species found in the intestines.
Thus, there are:
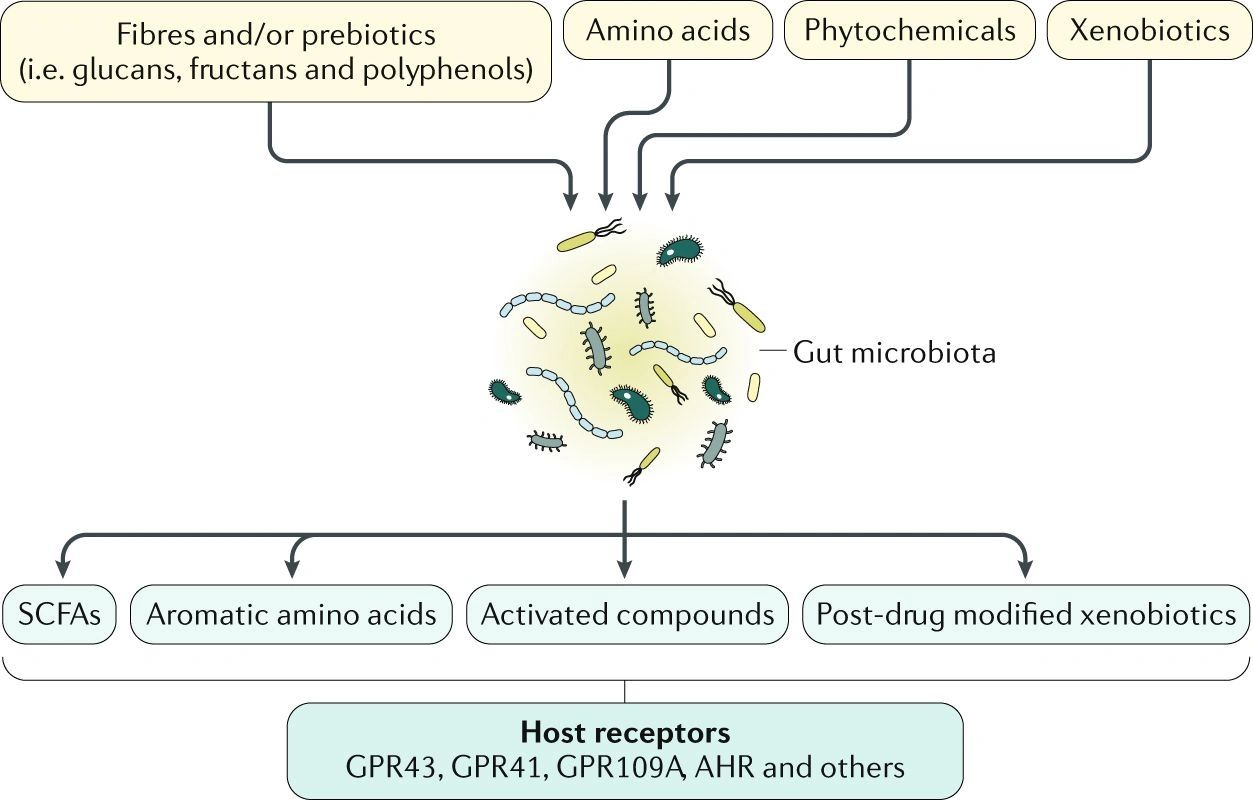
- Prebiotics that is, complex types of sugars (fibers) or non-digestible starches that nourish and provide favorable conditions for the growth of good bacteria (they are like fertilizers for intestinal bacteria).
They are found in:
- whole grains;
- onion;
- garlic;
- leek;
- artichoke;
- asparagus;
- dandelion;
- bananas;
- seaweed (Chlorella, Spirullina);
- beans;
- chicory and oats drink;
- dietary supplements with prebiotics.
We should bear in mind that high prebiotic (fiber) food consumption, mainly when suddenly introduced, can increase the production of gas (flatulence) and bloating.
Persons with gastrointestinal sensitivities, such as irritable bowel syndrome, can incorporate certain foods in small amounts and slowly determine individual tolerances first.
Tolerance can be increased with fewer side effects in the case of continuous use.
If there is no such sensitivity, it is vital to increase the intake of fiber because a low fiber diet can not only reduce the amount of beneficial microbiota but can also increase pathogenic bacteria (by lowering the intestinal pH).
2.probiotics, i.e., live bacteria (for example, Lactobacilli species) favorable to the gut flora.
Found in:
a. Fermented foods, such as:
- kefir;
- yogurt (preferably homemade, unpasteurized, which retains essential concentrations of live cultures);
- pickled vegetables;
- tempeh;
- kombucha tea;
- kimchi;
- miso;
- cabbage;
b.food supplements with probiotics.
Preferably, the regulation of the intestinal flora should take place through the diet, and nutritional supplements with pre- and probiotics should be administered only in certain particular situations (children, the elderly, antibiotic therapy).
Conclusion
The microbiome is a complex living environment in which the relative abundance of species will fluctuate daily, weekly, and monthly depending on diet, medications, activities, and a variety of other exposures to the setting.
Nevertheless, scientists are still in the early stages of understanding the significant role of the microbiome in human health and the extent of the problems that may arise as a consequence of the disruption of healthy interactions between the microbiome and its host.
So we're hosting millions of different microbes. As we understand more about them, how they interact with each other or with our body, we will find out how we can take care of this complex and invisible world that shapes our identity, health, and well-being.
Studies suggest that people with several disorders can recover faster if there is increased awareness of the intestinal microflora.
Thus, people with diabetes and gastrointestinal disease usually have fewer bacteria in their intestines. Likewise, there are prospects for depression, Parkinson's disease, Alzheimer's disease, and non-communicable chronic diseases using pro-and prebiotic products and even intestinal microflora transplantation.
In addition, pregnant women who took products that activate intestinal flora during pregnancy had a decreased risk of developing gestational diabetes.
References
1. "The Microbiome," Harvard T. H. Chan School of Public Health, available at https://www.hsph.harvard.edu/nutritionsource/microbiome/ [accessed April 6, 2018].
2. Ursell, L. K., Metcalf, J. L., et al., "Defining the human microbiome," in Nutrition Reviews, vol. 70, supplement 1, August 2012, pp. S38 – S44.
3. Morowitz, M. J., Carlisle, E., and Alverdy, J. C., "Contributions of intestinal bacteria to nutrition and metabolism in the critically ill," in The Surgical Clinics of North America, vol. 91, no. August 4, 2017, pp. 771-785.
4. Jandhyala, S. M., Talukdar, R., et al., "Role of the normal gut microbiota," in World Journal of Gastroenterology, vol. 21, no. August 29, 2015, pp. 8787-8803.
5. Canny, G. O., and McCormick, B. A., "Bacteria in the intestine, helpful residents or enemies from within?", In Infection and Immunity, vol. 76, no. August 8, 2008, pp. 3360–3373.
6. Galland, L., "The gut microbiome and the brain," in Journal of Medicinal Food, vol. 17, no. 12 December 2017, pp. 1261–1272.
7. Kuang, Y. S., Lu, J. H., et al., "Connections between the human gut microbiome and gestational diabetes mellitus," in GigaScience, vol. 6, no. 8, August 1, 2017, pp. 1–12.
Scientifical Approach on the Human Microbiome
Introduction
Genetic sequencing has wholly changed our comprehension of the tree of life and the position of humans inside it.
The introduction of the DNA sequencing method of Sanger in 1977 and the DNA amplification process of polymerase chain reaction (PCR) in 1983 led to a rise of genetic data that assessed the phylogeny of humans and the great apes, dismissed the biological definition of race in humans, and reconstructed the world's civilizations.
The diverse microbial species that inhabit the human body, denominated as the human microbiome, constitute a large amount of genetic and functional diversity that far exceeds that of our own nuclear and mitochondrial genomes).
Increased recognition of the role of microbiomes in the functions of basic host life, disease etiology, and even speciation, disputes traditional views on the definition of biological species and raises the question as to whether or not ancient human microbiomes would also be examined to address broader issues in human evolution.
This study will address the symbiosis and their microbiomes and will examine new developments in ancient microbiome research in the field of research. They suggest that we can only truly appreciate what it means to be human by studying our microbiomes today and in the past, too.
The Human Microbiome
Collectively, the human body's microorganisms contain an enormous amount of bacteria. It has been recognized since the late 1970s that the number of bacterial cells in and on the human body exceeds at least an astronomical amount the number of human cells.
More recently, in 2010, it was reported that our ' accessory genome ' estimated number of unique bacterial genes exceeds the number of our genes by a margin of 150.
While the bacteria are 1,000 times smaller than human cells, they nevertheless make up about 2 percent of the body mass (1.5 kg), making them collectively equal in size to the human brain (1.4 kg) or the liver (1.6 kg), leading some to draw attention to our inhabitant microbes as an entire human organ.
Conversely, the human-microbial bond was also equivalent to that of a superorganism, a colony of bees, or a holobiont, such as a coral reef.
A holobiont is an agglomeration of a host and the numerous other species that live in or near it, forming a distinct ecological unit. A holobiont's components are individual organisms or bionts, whereas the hologenome is the aggregate genome of all bionts.
Recognizing the need for a collective term referring to the large number of relatively unexplored and mostly unnamed microorganisms populating the human body, Joshua Lederberg coined the term microbiome in 2001 to "mean the ecological population of commensal, symbiotic and pathogenic microorganisms that share our body space and have all but been overlooked as determinants of health and disease.
The concept microbiome has already tended to be used more stringently, referring to the cumulative molecular (especially genomic) data obtained from a microorganism population, rather than the microorganisms themselves, resulting in some research misunderstanding.
For the specifics of this research, the word microbiome has been used as an ecological population of microorganisms following its original published description by Lederberg, the concept microbiota as a synonym shall also be employed.
Subsequently, the word hologenome is used to refer to the host's collective fenomas and its microbiome. Finally, to refer to combined genomic and proteomic data gathered from an environmental sample, the terms metagenome and metaproteome will be employed.
In tandem with significant technological developments in microbiology and molecular methods, these concepts have evolved to investigate complex microbial systems.
Recent technical advances in microbiome research
Early studies of human-associated microbes focused on individual bacterial species and strains being isolated and cultured. This leads to a further explanation and naming of hundreds of human microbiome species using those strategies.
Nonetheless, culture-free molecular analyzes of prokaryotic 16S ribosomal RNA (rRNA) and the 16S rRNA gene using methods first introduced by Carl Woese and Norman Pace show that at least 60-80 percent of bacteria inhabiting the human body can not be cultivated in a laboratory.
New molecular methodologies have been developed to access these unculturable members, particularly terminal restriction fragment length polymorphism (T-RFLP) molecular fingerprinting, single target PCR, multiplex PCR, quantitative PCR (qPCR), fluorescent in situ hybridization (FISH), checkerboard hybridization, and 16S microarrays based on rRNA, inter alia.
The most effective of these methods merged PCR amplification of 16S rRNA genes from a mixed microbial sample using universal bacterial primers, followed by cloning PCR products into a plasmid vector, transformation of the plasmids into competent Escherichia coli cells, plating and colony selection, plasmid purification, and sequencing of up to several thousand clones by Sanger.
Such sequences could then be grouped into distinct phylotypes which correspond approximately to species of bacteria by proximity (usually 97.0 percent or 98.5 percent sequence similarity).
This approach was successfully used in 2010 to classify 600 predominant members of the human oral microbiome. However, again, it is not feasible to characterize uncommon or less prevalent oral morphological traits using this method given the high-cost, labor-intensive, and low-performance nature of the cloning and sequencing phases.
The advent of NGS has influenced microbiome investigations by removing the cloning requirement and allowing millions of 16S rRNA gene sequences to be obtained at a fraction of the cost per base compared to conventional techniques.
Notwithstanding shorter reading lengths and shifting focus to hypervariable regions of the individual 16S rRNA gene rather than the entire genome, the expanded sequencing depth has identified a wide range of low-abundance taxa within the human microbiome, increasing the established taxonomic diversity of the oral microbiome alone by more than one order of magnitude.
Latest generation sequencing has also enabled the combined analysis of all genes and genomes within a microbiome, microbiome metagenomics, feasible as well. This has allowed microbiome research to move further than taxonomic ' who's there ' questions to practical ' what they're doing. '
Besides, the amplification of the single-cell genome and bioinformatics advances in sequence aggregation from metagenomic datasets allow complete genome reconstruction of unculturable taxa, offering our first glimpse of the potential role of these mysterious organisms.
Concerning primitive microbiomes, NGS provides two more benefits compared to previous methods. Firstly, the technique can be applied to dead cells as a culture-free method, and secondly, NGS is designed for fragmented DNA inside the range of sizes typical of ancient DNA (< 300bp).
Advances in metagenomic techniques in the field of metaproteomics are also being compared. Previously, microbial protein detection relied primarily on antibody-based procedures, often tied to gel electrophoresis (e.g., Western Blotting). Proteomics-based approaches advanced through the use of 2D (pI versus MWt) comparative gels with specific locations eluted and examined by soft simultaneous mass spectroscopy (MS / MS) ionization.
Such ' top-down ' approaches are gradually being replaced by ' bottom-up ' solutions, also known as shotgun proteomics, which perform mass spectrometric analyses of peptides from the complete protein extract's enzymatic digestion.
These bottom-up methods allow for higher performance and are also more applicable to primitive protein research because they do not involve intact molecules of protein.
Such advanced molecular methods provide unparalleled insights into the activity and malfunction of human microbial populations and provide a framework for understanding the role of gut microbiota in human health and disease.
Human microbiome function and dysfunction
Since the early 2000s, numerous studies have used a range of molecular methodologies to investigate the structure and function of the human microbiome.
A significant boost to these activities began in 2008, following the launch in Europe of the National Institutes of Health Human Microbiome Project (HMP) in the United States and the Human Intestinal Tract Metagenomics (MetaHIT) project.
Although human-associated microorganisms were historically seen at best as passive commensal tag-longs or irritations to be rinsed or flossed away, we now recognize that human oral, intestinal, skin and uritogenital microbiota play critical roles in sustaining host health by supporting essential functions in digestion and metabolism, vitamin development, and immune system education and maintenance.
Nonetheless, when threatened by poor diet, illness, stress, antimicrobial drugs, and other environmental disturbances, human microbiome ecology can migrate from a mutualistic to a dysbiotic state, leading to local and systemic diseases as diverse as obesity, type II diabetes, irritable intestinal disease and colon cancer, periodontal and dental decay, atherosclerosis and endocarditis.
The healthy human microbiome also contains several common, but highly severe, opportunistic pathogens, such as Streptococcus pneumonia, Haemophilus influenzae, Neisseria meningitidis, Clostridium difficile, Propionibacterium acnes and Staphylococcus aureus, some of which are implicated in hospital and community infections and present a particular risk to the elderly and the immune system.
Increasingly, multiple antibiotic-resistant compounds are overwhelmingly detected in normal oral and gastrointestinal microbiota in healthy individuals.
This appears to suggest that the use of antibiotic therapy may affect non-clinical goals, either through the direct clinical implementation or via indirect growth stimulation or prophylactic treatment in livestock, and may result in long-term intrinsic reservoirs of antibiotic resistance.
Furthermore, antibacterial remedies can perturb healthy bacterial communities themselves, resulting in further complications.
For example, wide-spectrum antibiotic therapeutic courses are known to disrupt the gut and uritogenital microbiota, where they can induce antibiotic-associated colitis and bacterial vaginosis, alike.
As a result, the interest in probiotic and prebiotic remedies for the treatment of disrupted microbiomas is increasing, but lack of basic knowledge about what defines a healthy microbiome, as well as a greater understanding of the propagation and development of healthy microbiota, are hindering factors in the development of these therapies.
While significant efforts have been made to classify healthy gut and oral microbiomas, recent studies on rural, non-Western populations have expressed concerns as to whether the microbiota we currently identify as natural has been influenced by recent effects of the modern Western diet, hygiene, antibiotic prominence, and lifestyle.
The industrial revolution has dramatically reduced our contact with natural environments and has fundamentally changed our relationship with food production.
The human oral and gut microbiomes, found at the entry point of our food and the locus of food digestion, have developed in circumstances of regular exposure to several environmental and zoonotic microbes which are no longer in play in today's globalized food chain.
In addition, the food itself has shifted from the wild natural products eaten by our hunter-gatherer ancestors to today's industrialized supermarkets packed with an excess of highly processed Western foods containing chemically refined levels of sugar, oil, and salt, not to include antimicrobial preservatives, petroleum-based dyes, and many other additives.
This dietary shift has changed the pressure of selection on our microbiomas. For example, disorders such as dental caries and gum disease are defined under the ' ecological plaque hypothesis ' as oral ecological disasters of cultural and eating habits.
Although it may be already evident that the human microbiome plays a crucial role in making us human, in maintaining us healthy, and in making us sick, we know remarkably few of the diversity, variation, and evolution of the human microbiome both recently and in the past.
Instead, many questions remain:
When and how did our bacterial populations become distinctly human?
And what would that imply for today and the future of our microbiomes?
How do we develop and distribute microbiomas, and to what extent are our cultural traditions and installed environments affected by this?
How have contemporary western diets, hygiene standards, and antibiotic prominence affected the function of ' normal ' microbiomas?
Are we still in mutual symbiosis with our microbiomes or are the so-called ' civilizational diseases ' – heart disease, obesity, type II diabetes, asthma, allergies, osteoporosis – testimony that our microbiomes are not ecologically balanced and dysbiotic?
Who are the representatives of the human microbiome on an even more in-depth level, how did they happen to inhabit us, and how long would they have been there? Who is ' our microbial self '?
Research of rural and indigenous communities, and crowdfunding initiatives such as the American Gut, the Earth Microbiome Project, and uBiome aim to describe current microbiomes over a variety of modern settings.
Moreover, even the most thorough analysis of modern microbiota can give a limited perspective into pre-industrial microbiomas.
By comparison, direct study of ancient microbiomes from isolated places and historical periods in the past might provide a fascinating perspective of the coevolution of microbes and organisms, host-microbial ecology, and improvement of the state of human health over time.
Ancient Microbiome Research
Upon death, the human microbiome's ecosystem develops radically through the decomposition of soft tissue.
Apart from frozen and mummified organisms, only two microbiomes typically produce substrates that survive after death in geological conditions under favorable conditions: the fecal content of the intestinal microbiome can be desiccated or mineralized to produce coprolites, and the oral microbiome dental plaque calcifies in situ across the entire life so that it is already in a semi-fossilized state.
The ability to examine ancient microbiomas directly by coprolites and dental calculus empowers us to redefine past human health issues within a much broader context that involves not only the analysis of compulsory infectious pathogens but also the transportation levels and risks posed by endemic and latent pathogens, as well as the safety and resilience of the microbial population.
Coprolites
Coprolites are mineralized or desiccated feces. Coprolites dating back to 270 Ma (millions of years ago) were found during the Paleozoic Period, and many prominent specimens of dinosaur coprolites are known from the Cretaceous Age ca. 145–66 Ma.
Feces contain an unbelievable number of microorganisms – more than one billion viable bacterial cells per milligram of stool. As such, feces are extremely biologically active and usually rapidly decompose, except in uncommon circumstances.
The immediate post-deposition environment is critical for the biomolecular-level survival of coprolite. Latrine deposits and soils that are important for the study of diets and parasites undergo the same taphonomic challenges.
Nonetheless, calcareous deposits, such as those preserved from latrine drain pipes in Pompeii, can provide a better conservation condition, particularly if feces particles are quickly encrusted with mineral scale, thus mitigating both the decomposition process and the environmental pollution process.
Dental Calculus
Dental calculus represents a calcified bacterial biofilm formed on the teeth's surfaces and is present in all human populations, as well as Miocene primates, Neanderthals, wild chimpanzees, and several animals.
The occurrence of dental calculus among humans, both in the past and today, when dental care could not be available, is almost pervasive amongst adults aged 30, and our experience has shown that it is not unusual to find dental calculus deposits above 100 milligrams in the farming population archeological excavations.
Besides being pervasive, and comparatively readily available, dental calculus is also a rich source of ancient biomolecules with extracted DNA outputs of up to three magnitude orders higher than the same individual's bone or dentine.
Other potential sources of ancient microbiome data
- Historic medical specimens
- Mummified human remains
- Secondary deposition in bone
References
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4312737/
Ancient human microbiomes
Christina Warinner, Camilla Speller, Matthew J. Collins, and Cecil M. Lewis, Jr.
Probiotics & Prebiotics

The Gut Microbiota
Additional Information
There is a whole new scientific vocabulary and an explosion of GUT knowledge that increases each year steadily.
Stomach symptoms have increased over the past two decades, and many people are diagnosed with diarrhea, constipation, vomiting, coeliac disease, malabsorption of fructose, leaky gut, intestinal permeability, Crohn's disease, ulcerative colitis, SIBO (overgrowth of small intestinal bacteria), malabsorption of fat, indigestion, and cancer of the alimentary canal.
Systemic diseases involving heart disease, diabetes, cancer, and autoimmunity have also undergone a steady rise.
Autoimmunity is a broad term comprising, most prominently, one hundred plus disorders, multiple sclerosis, type 1 diabetes, lupus, hashimotos, sepulchers, add-ons, Parkinson's and rheumatoid arthritis.
Previously, a majority of people reported to their physicians, identified their symptoms, diagnosed illness, and prescribed medication and confirmed that their wellbeing or symptoms had nothing to do with their diet.
The food we bring into the body affects every cell and its entire biochemistry, thus affecting every system and every organ.
Not only do we acknowledge that the food we eat will give us the strength and foundation of the body, but now we realize that any food we eat will influence our DNA.
The GUT has a population of bacteria, viruses, fungi, mushrooms, and yeasts. Those kinds of microbes live symbiotically with us, help us digest, prevent infection, make vitamins, amino acids, fats, etc.
The health of our Microbiota is essential to wellbeing. Such bugs live symbiotically with us, helping us digest, combat diseases, produce vitamins, amino acids, fats, etc.
Our modern lifestyle, food contaminated with pesticides, pollutants in nature and our homes, heavy antibiotic use, food consumption, refined, packaged foods, inadequate sleep and hygiene, insufficient sunlight, all contribute to dysbiosis (sickness). We get sick when there's dysbiosis in the GUT and Microbiota.
While most of us have become aware of probiotics due to physician and pharmacist recommendations, prebiotics are less well known.
There are significant differences between the two, and there are also different health benefits to the human body.
Although probiotics have proved effective in handling some gastrointestinal issues, they do not have the same power as prebiotics.
Therefore, probiotics identify living microorganisms that colonize our digestive tract (mostly bacteria). Such microorganisms help us digest food, a cycle in which food consumes nutrients and vitamins, creates vitamins B and K, and prevents the production of other microorganisms that could damage our health.
Probiotics are found both in the form of dietary supplements, which we can purchase from pharmacies, but are also present in certain types of fermented foods for which: sour cabbage, pickles yogurt, kefir, soft fermented cheeses bread dough.
To enjoy the benefits of probiotics obtained from food sources, these bacteria have to be alive and healthy throughout the food's shelf life to survive digestion and ultimately colonize the gut. Therefore, by regulating the harmful bacteria, they help maintain the health of the digestive system.
On the other hand, to help maintain them, prebiotics are often described as probiotics for "food." Prebiotics are soluble fibers and other groups of carbohydrates, such as starch and sugars called oligosaccharides that are fermented by bacteria.
Prebiotic sources include asparagus leeks from bananas potatoes, onions, garlic chicory, flakes from artichoke oatmeals.
Prebiotics have many benefits in helping to better digest minerals such as calcium and magnesium, shielding the body from bacterial infections, combating bowel pain, diarrhea, and irritable bowel syndrome.
So we have to be careful to add a variety of vegetables and as many fibers into our diet to maintain the health of the digestive tract that determines the health of the entire body.
What are prebiotics?
Prebiotics are non-digestible carbohydrate compounds. Specifically, they are a type of fiber, best known as oligosaccharides. As a mildly amusing detail, all prebiotics are fibers, but not all fibers are prebiotics.
The non-digestible components of foods are prebiotic fibers.These are known to travel undigested through the small intestine before they enter the colon, where the intestinal microflora (bacteria) ferment them. We promote the growth and development of bacteria in the digestive system when fermented, which can provide potential health benefits.
Prebiotics are different from probiotics.
In short, probiotics in the digestive tract are good bacteria. These support the process of digestion and have other potential health benefits. They are quite frequently found in fermented foods like pickled cabbage and kefir. Prebiotics aren't crops that exist. These are fibers that are non-digestible and nourish probiotic bacteria.
Why should we consume prebiotics?
There are many good reasons why prebiotics should become a daily part of your diet. Research has shown that higher prebiotic intake can offer the following health benefits:
- Healthier gut because beneficial bacteria prevent harmful bacteria from entering the gastrointestinal tract
- Ensure proper functioning of the immune system
- Weight loss risk by controlling appetite
- Reduction of inflammation associated with inflammatory bowel disease
- Reducing the risk of cardiovascular disease by regulating cholesterol levels
- Reduce the risk of type 2 diabetes by improving the rate of glucose uptake
- Improvement and strengthening of the digestive system
- Increased availability of minerals in the body
- Decreased risk of colon cancer
For the most part, fiber ingested by the diet is the primary source of prebiotics-specifically certain types of fruits, vegetables, and carbohydrates. Inulin, which is a soluble dietary fiber, comes from raw garlic, asparagus, and onions.
If you're worried you don't have enough prebiotics in your diet, supplements in a concentrated form may provide the required protection. You can also make sure of your daily intake of prebiotics and some food.
For the most part, fiber ingested by the diet is the primary source of prebiotics-specifically certain types of fruits, vegetables, and carbohydrates. Inulin, which is a soluble dietary fiber, comes from raw garlic, asparagus, and onions.
Indeed, it has been demonstrated that inulin is prebiotic. The difference between certain difficult-to-digest fibers is that inulin is a water-soluble fiber. Also, it is found in onions, leeks, garlic, asparagus, artichokes, and many other foods.
If you don't think you consume enough prebiotics in your diet, inulin is an easy-to-mix prebiotic powder supplement. Just add 1-2 cups of 5 g of water in a shake or smoothie once a day.
The best food sources of prebiotics
Vegetables are the best sources of prebiotics, but because the fibers break down through cooking, it's good to eat them raw to get the best effect. Other healthy prebiotic sources are whole grains, green bananas, or potatoes.
More prebiotics can be consumed in the diet through isolated carbohydrates (known as galactooligosaccharides and transgalactooligosaccharides).
The following foods are worth considering for an increase in the intake of prebiotics:
Legumes (such as beans, lentils, and peas)
- Whole wheat
- Oats
- Honey
- Boiled and chilled potatoes
- Root of raw chicory
- Inulin
- Raw garlic
- Raw term
- Raw or cooked onions
- Raw asparagus or cooked al dente
- Acacia gum
- Green bananas
- Whole psyllium shells
Conclusion
Prebiotics are non-digestible sources of fiber, and inulin is a water-soluble variety.
Prebiotics are needed to develop healthy (beneficial) bacteria that support both the digestive system and other functions, such as the immune system.
Prebiotics may be obtained from dietary sources, mainly fruit and vegetables, but prebiotic supplements are available to ensure that the uptake meets the daily requirement.
Copyright © 2019 Louisiana Agriculture Preservation Society - All Rights Reserved.
Powered by GoDaddy Website Builder